Location and Capabilities
Menarini Biotech is a fully integrated CDMO specializing in first-in-class biotherapeutic development and manufacturing of biologics. Established in 2003, Menarini Biotech’s mission is to provide high-quality, bespoke end-to-end services for biopharm companies from cell line development to GMP manufacturing of drug candidates. Our Quality System complies with AIFA/EU. Menarini Biotech works also in accordance with highest ISO certifications such as ISO-14001, ISO-45001 and ISO-50001.
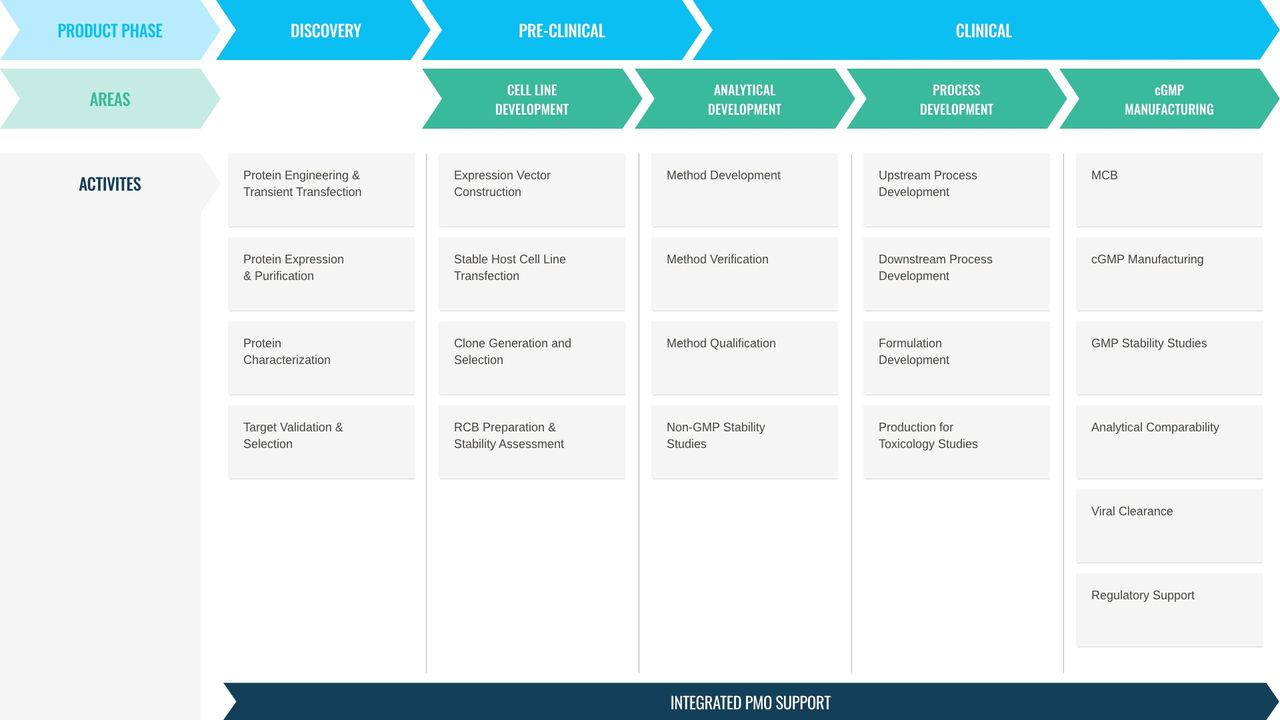
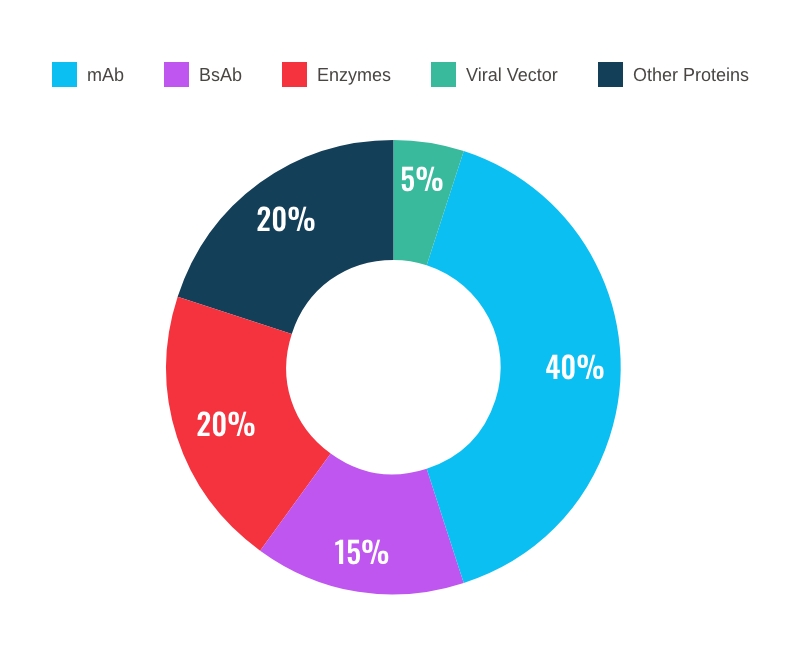
We offer twenty years of experience in lead candidate selection and optimization of cell lines, process and analytical development, process optimization as well as drug substance and drug product manufacture from early clinical to commercial phase.
Our track record
1998-2023
POMEZIA (ROME, IT)
The Headquarters of Menarini Biotech are located in the greater Rome area (Italy) in a cutting-edge pharmaceutical campus. The Italian site boasts an area dedicated to process development, an area dedicated to large scale production activities, including all the necessary support services (warehouse, offices and utilities) and a separate area dedicated to quality control services.
The production of active substances for clinical trials I, II and III takes place in two segregated suites in class D clean room, with 2X1,000L and 3X200L bioreactors (UNI EN ISO 14001:2004; OHSAS 18001).
The purification steps take place in class C clean room (UNI EN ISO 14001:2004; OHSAS 18001) with pre-viral and post-viral suite separation.


