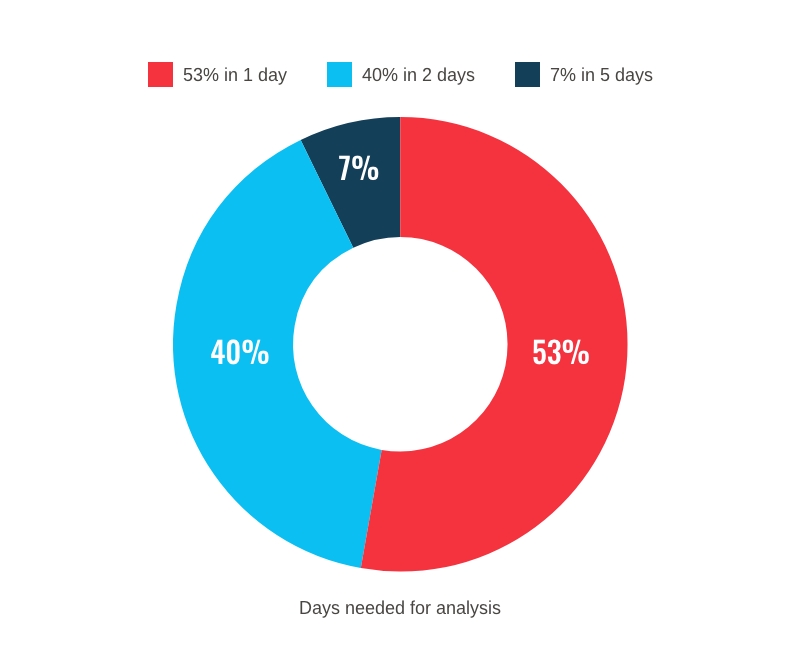
Our team is able to perform around 70 analytical techniques in a week using qualified instrumentation.
In addition, we are able to perform >50% of our analyses in a working day.
Quality Control in Menarini Biotech certifies the quality of the manufacturing process according to GMP regulations, ensuring compliance with the correct analytical specifications and all quality standards. Our services include :

Our team is able to perform around 70 analytical techniques in a week using qualified instrumentation.
In addition, we are able to perform >50% of our analyses in a working day.

QC Analysis Timing