Bioassays Portfolio
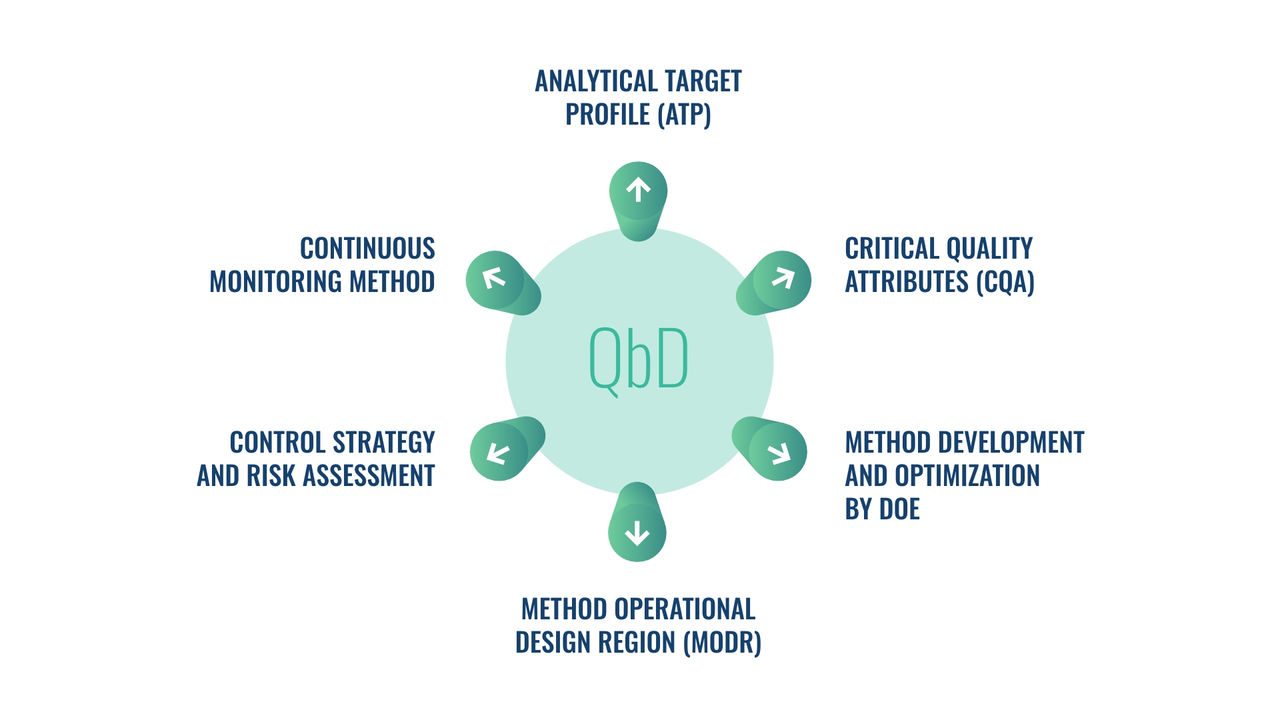
A specialized team will support you from cell bank preparation for analytical purposes (WCB), identification of the most appropriate method based on your product and Mechanism of action, and then method development and optimization up to validation.
The DOE is applied from development to validation to make your results more robust and through statistical analysis we support stability data and batch to batch comparability.
You will be able to have platforms to verify the mechanism of action of your product including (and not limited to):
- Complement dependent cytotoxicity (CDC) assay
- Antibody dependent cytotoxicity (ADCC) assay
- Cell-based Competition assays by SPR technology